Embark on an enlightening journey into the realm of chemistry with Calculating Percent by Mass/Volume Chem Worksheet 15-2. This comprehensive resource delves into the fundamental concepts and practical applications of percent by mass and percent by volume calculations, equipping you with the knowledge and skills to master this essential aspect of chemical analysis.
Throughout this worksheet, you will explore real-world scenarios where these calculations play a crucial role, from preparing solutions and determining concentrations to analyzing complex mixtures. With clear explanations, step-by-step guides, and ample practice problems, you will gain a thorough understanding of percent by mass and percent by volume, empowering you to confidently navigate the complexities of chemistry.
Definitions and Concepts
Percent by Mass:The ratio of the mass of the solute to the mass of the solution, expressed as a percentage. It measures the amount of solute present in a given mass of solution.
Percent by Volume:The ratio of the volume of the solute to the volume of the solution, expressed as a percentage. It measures the amount of solute present in a given volume of solution.
Calculations: Calculating Percent By Mass/volume Chem Worksheet 15-2
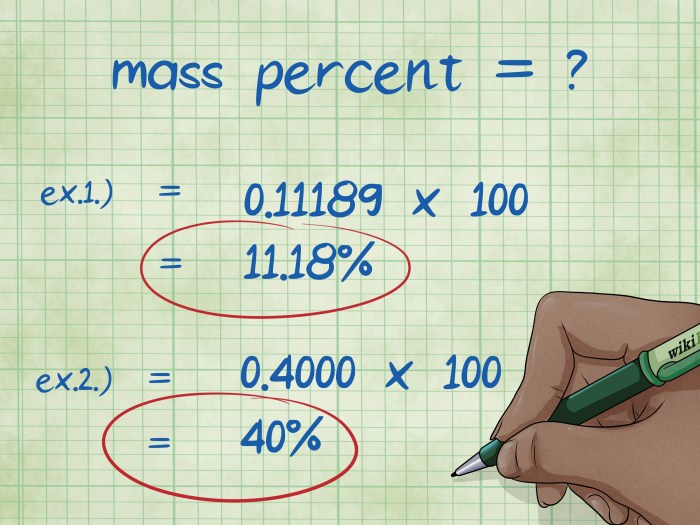
Percent by Mass, Calculating percent by mass/volume chem worksheet 15-2
Steps:
- Determine the mass of the solute (m solute).
- Determine the mass of the solvent (m solvent).
- Calculate the mass of the solution (m solution) as m solute+ m solvent.
- Calculate the percent by mass using the formula: % by mass = (m solute/ m solution) x 100%
| Solute Mass (g) | Solvent Mass (g) | Solution Mass (g) | Percent by Mass |
|---|---|---|---|
| 10 | 90 | 100 | 10% |
Percent by Volume
Formula:% by volume = (V solute/ V solution) x 100%
| Solute Volume (mL) | Solvent Volume (mL) | Solution Volume (mL) | Percent by Volume |
|---|---|---|---|
| 20 | 80 | 100 | 20% |
Clarifying Questions
What is the difference between percent by mass and percent by volume?
Percent by mass expresses the amount of solute present in 100 grams of the solution, while percent by volume represents the amount of solute present in 100 milliliters of the solution.
How do I calculate percent by mass?
Percent by mass = (Mass of solute / Mass of solution) x 100%
What are some applications of percent by mass calculations?
Percent by mass calculations are used in preparing solutions, determining concentrations, and analyzing mixtures.