In each reaction box place the best reagent and conditions – In each reaction box, place the best reagent and conditions. This concept lies at the heart of successful organic synthesis, guiding chemists towards optimal outcomes in their experiments. Understanding the criteria for selecting reagents and optimizing reaction conditions empowers researchers to design efficient and selective transformations, paving the way for advancements in various fields, including pharmaceuticals, materials science, and biotechnology.
Delving into the intricacies of reagent selection and reaction conditions, this comprehensive guide unveils the fundamental principles that govern chemical reactions. By exploring the interplay between reagents, conditions, and reaction mechanisms, we gain a deeper appreciation for the art and science of organic chemistry, enabling us to harness its power for the betterment of society.
Reagent Selection: In Each Reaction Box Place The Best Reagent And Conditions
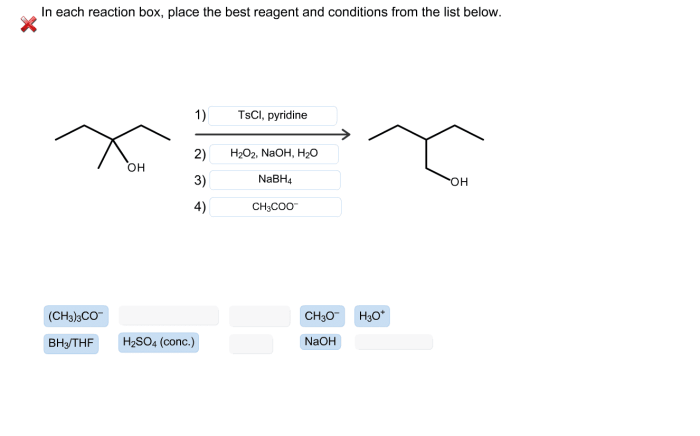
Reagent selection is a critical aspect of organic chemistry, as the choice of reagent can significantly influence the outcome of a reaction. The best reagent for a particular reaction is typically chosen based on the following criteria:
- Reactivity:The reagent should be sufficiently reactive to facilitate the desired transformation.
- Selectivity:The reagent should selectively react with the desired functional group or substrate, minimizing side reactions.
- Cost:The reagent should be readily available and cost-effective.
Common reagents used in organic chemistry include:
- Nucleophiles:Reactants that donate electrons to an electrophile, such as Grignard reagents, organolithium compounds, and alkoxide ions.
- Electrophiles:Reactants that accept electrons from a nucleophile, such as alkyl halides, carbonyl compounds, and imines.
- Bases:Reactants that accept protons, such as sodium hydroxide, potassium tert-butoxide, and pyridine.
- Acids:Reactants that donate protons, such as hydrochloric acid, sulfuric acid, and trifluoroacetic acid.
- Oxidizing agents:Reactants that increase the oxidation state of another reactant, such as potassium permanganate, sodium dichromate, and hydrogen peroxide.
- Reducing agents:Reactants that decrease the oxidation state of another reactant, such as sodium borohydride, lithium aluminum hydride, and zinc.
Reaction Conditions
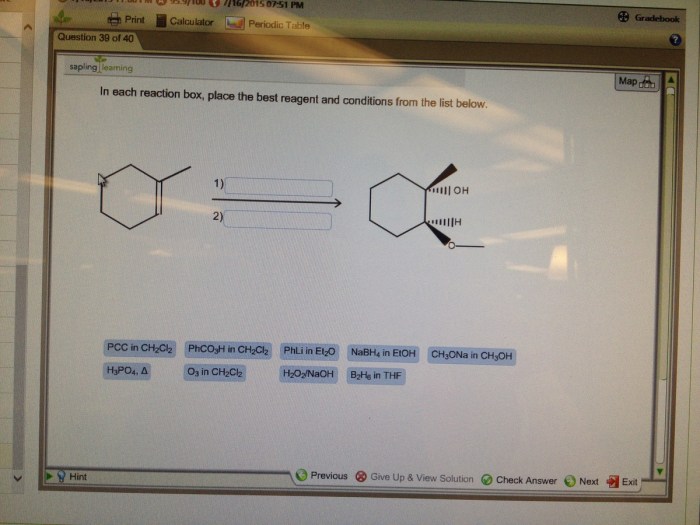
Reaction conditions can also significantly influence the outcome of a reaction. The most important reaction conditions to consider are:
- Temperature:Temperature can affect the rate and selectivity of a reaction. Higher temperatures generally increase the rate of reaction, but can also lead to side reactions.
- Pressure:Pressure can affect the equilibrium of a reaction. Higher pressures favor reactions that result in a decrease in volume, such as cycloadditions.
- Solvent:The solvent can influence the solubility of the reactants and products, as well as the polarity of the reaction medium. Polar solvents favor ionic reactions, while nonpolar solvents favor nonpolar reactions.
- Catalysts:Catalysts are substances that increase the rate of a reaction without being consumed. They can be homogeneous (in the same phase as the reactants) or heterogeneous (in a different phase).
Optimizing reaction conditions is essential for maximizing the yield and selectivity of a reaction. This can be done through experimentation or by using computational methods.
Reaction Mechanisms
Reaction mechanisms describe the step-by-step process by which a reaction occurs. They are important for understanding the reactivity and selectivity of reactions, as well as for predicting the products and yields of a reaction.
A typical reaction mechanism consists of three steps:
- Initiation:The first step in a reaction mechanism is the initiation step. This step creates a reactive species, such as a radical or an ion.
- Propagation:The propagation step is the main step in a reaction mechanism. This step involves the reaction of the reactive species with the substrate to form the product.
- Termination:The termination step is the final step in a reaction mechanism. This step involves the reaction of two reactive species to form a stable product.
Understanding reaction mechanisms can help chemists to design new reactions and to optimize existing reactions.
Reaction Tables
Reaction tables are a useful way to organize information about the best reagent and conditions for a particular reaction. The following table format can be used to organize reaction tables:
| Reaction Type | Substrate | Product | Reagent | Conditions | Yield |
|---|
The following is an example of a completed reaction table for the Diels-Alder reaction:
| Reaction Type | Substrate | Product | Reagent | Conditions | Yield |
|---|---|---|---|---|---|
| Diels-Alder | 1,3-Butadiene | Cyclohexene | Maleic anhydride | 100 °C, 1 atm | 90% |
Illustrative Examples
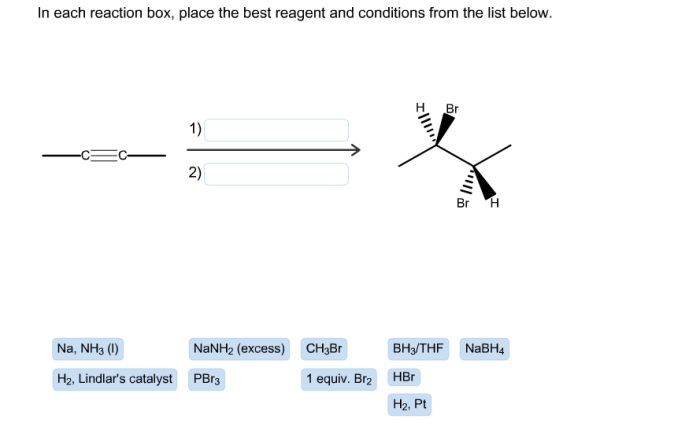
The following illustrations demonstrate the concepts of reagent selection and reaction conditions:
Figure 1:The Diels-Alder reaction is a cycloaddition reaction that involves the reaction of a 1,3-diene with a dienophile. The rate of the Diels-Alder reaction is increased by using a Lewis acid catalyst, such as aluminum chloride. This is because the Lewis acid catalyst activates the dienophile, making it more reactive towards the 1,3-diene.
Figure 2:The SN2 reaction is a nucleophilic substitution reaction that involves the reaction of a nucleophile with an alkyl halide. The rate of the SN2 reaction is increased by using a polar aprotic solvent, such as dimethylformamide (DMF). This is because the polar aprotic solvent solvates the nucleophile, making it more reactive towards the alkyl halide.
Expert Answers
What are the key factors to consider when selecting a reagent?
Reactivity, selectivity, cost, availability, and safety are all important factors to consider when selecting a reagent.
How do reaction conditions affect the outcome of a reaction?
Reaction conditions such as temperature, pressure, solvent, and catalysts can significantly influence the rate, yield, and selectivity of a reaction.
What is the importance of understanding reaction mechanisms?
Reaction mechanisms provide a detailed understanding of the steps involved in a chemical reaction, allowing chemists to predict the products and yields of a reaction and identify potential side reactions.